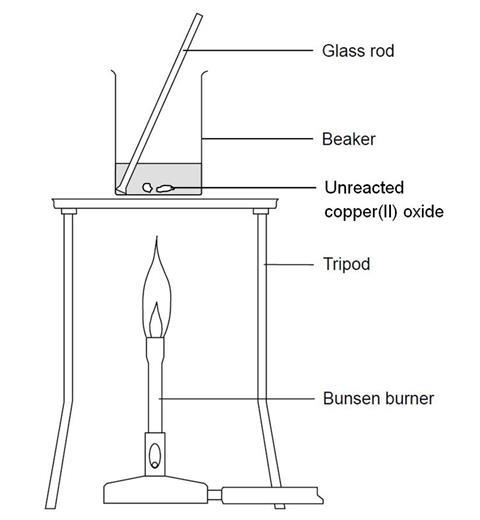
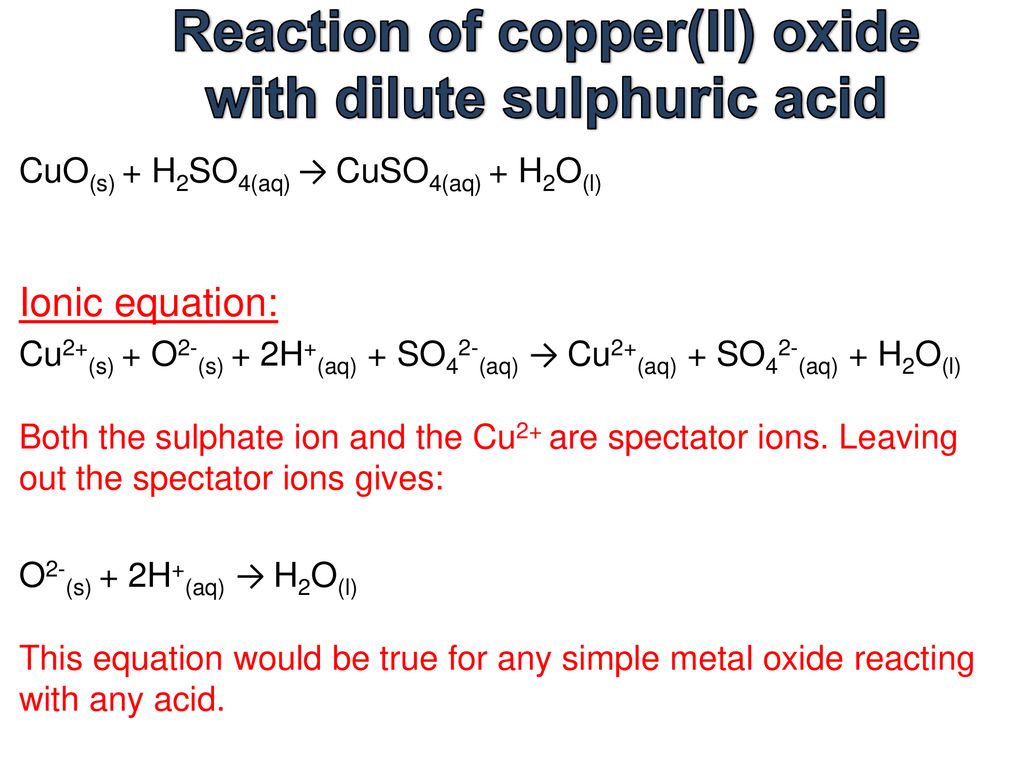
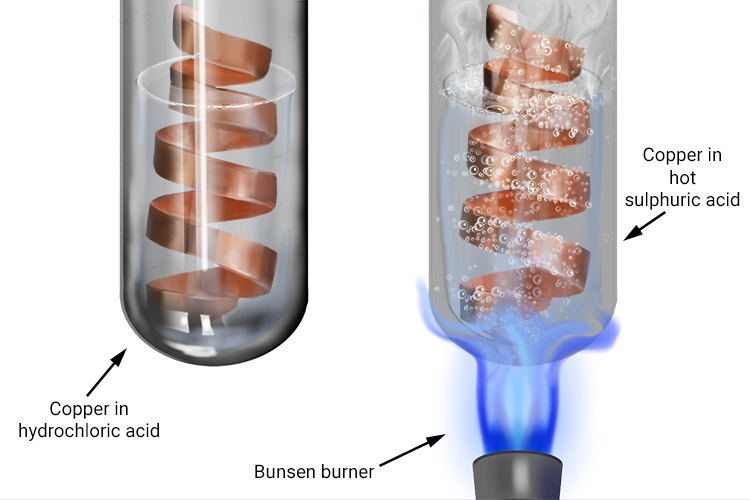
On heating copper turnings with conc. H2SO4 , a colourless gas with pungent smell is evolved which decolourises acidified KMnO4 solution. Identify the gas.

Predict reaction of 1n sulphuric acid with copper lead and iron - Chemistry - Electrochemistry - 13647999 | Meritnation.com
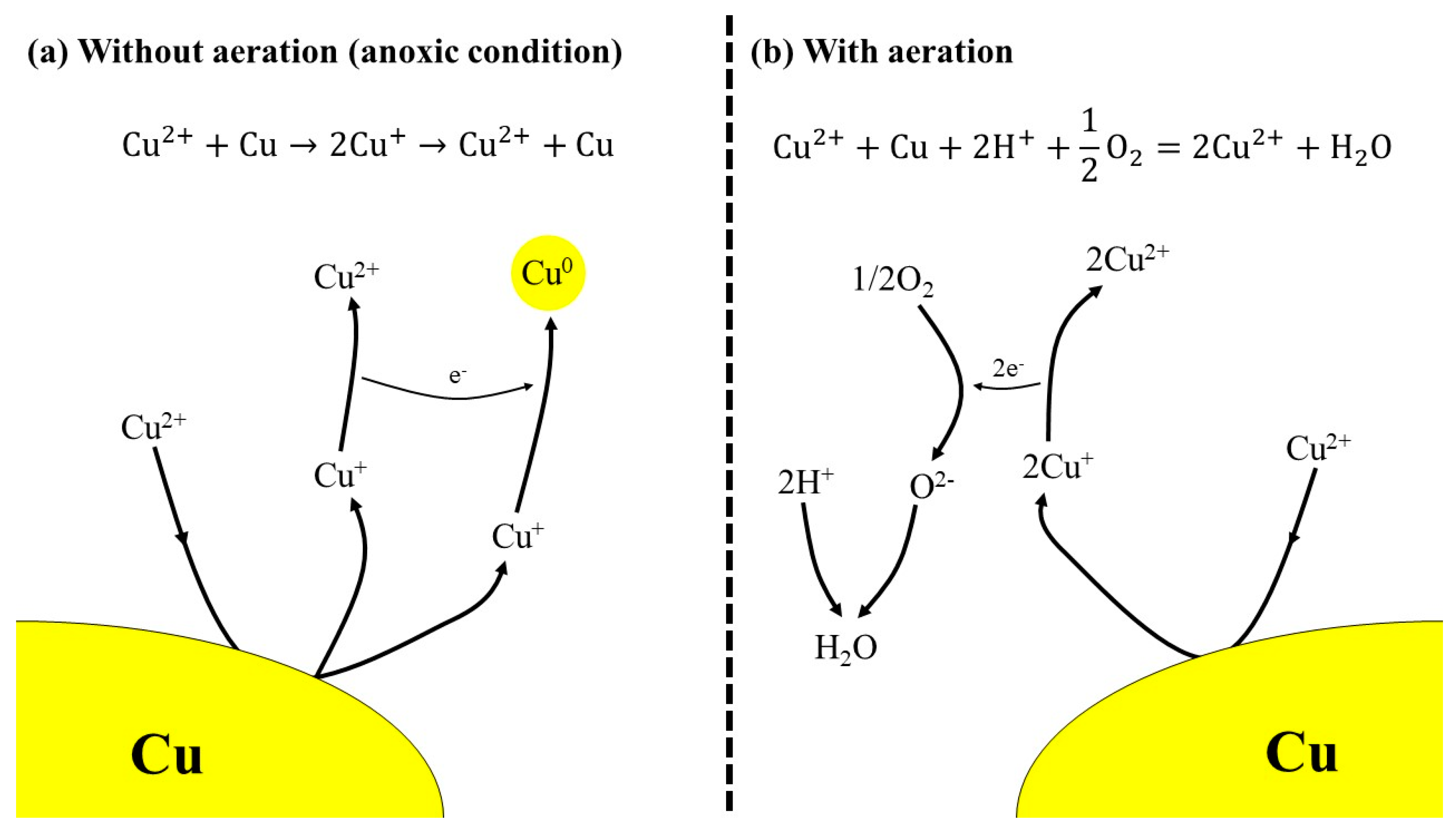
Metals | Free Full-Text | Improvement of Copper Metal Leaching in Sulfuric Acid Solution by Simultaneous Use of Oxygen and Cupric Ions
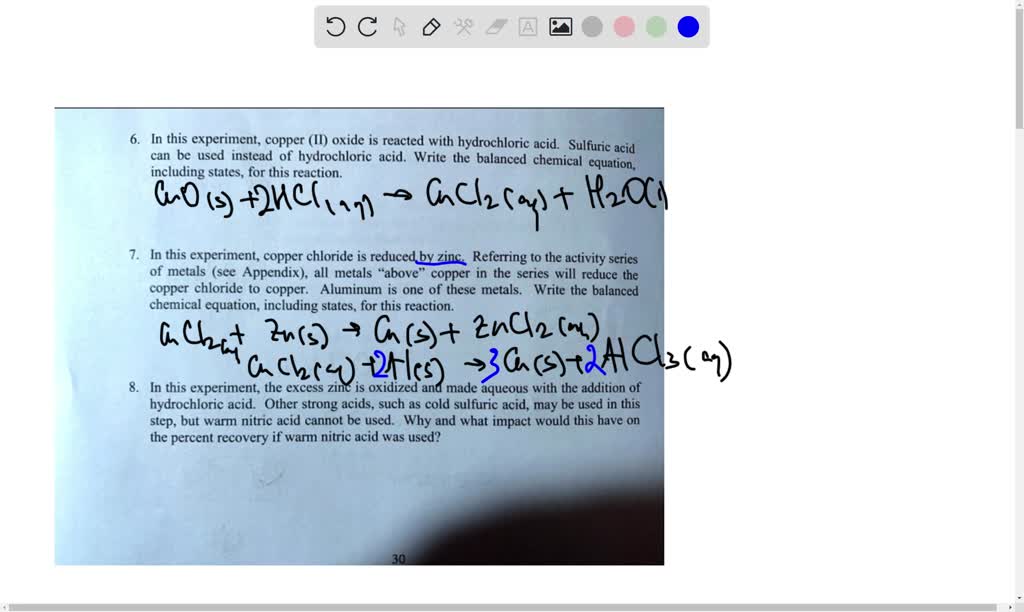
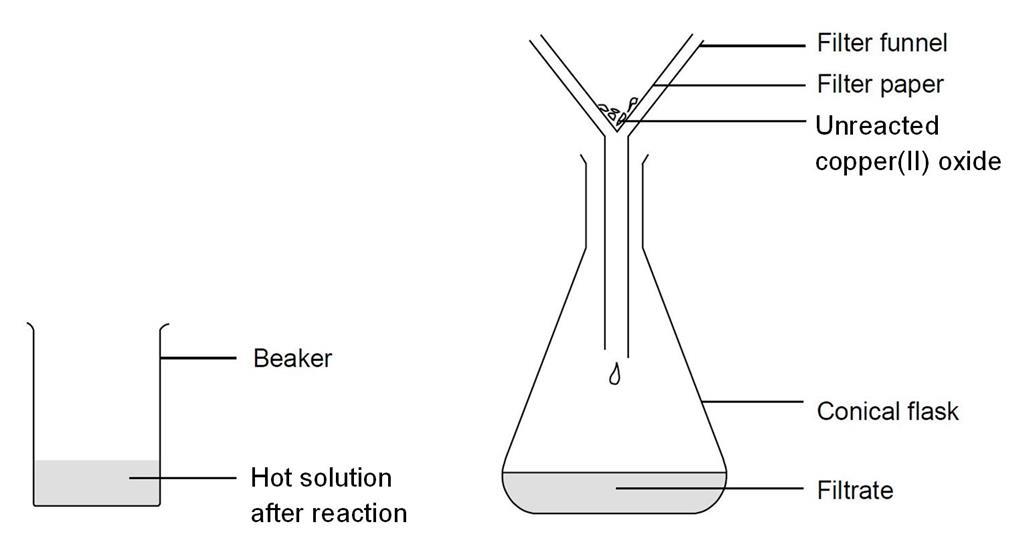
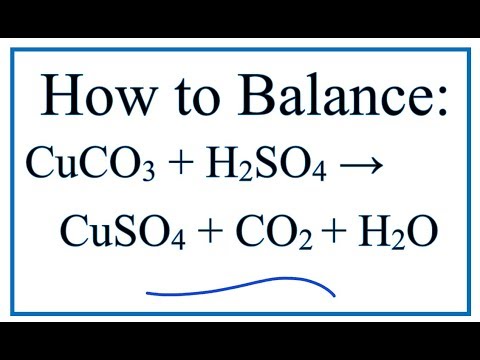
SOLVED: In this experiment, copper (II) oxide is reacted with hydrochloric acid. Sulfuric can be used acid instead of' hydrochloric acid Write the balanced chemical equation; including states, for this reaction In

what happens when dilute sulphuric acid is poured on a copper plate ? write chemical equation. - Brainly.in

Metals | Free Full-Text | Improvement of Copper Metal Leaching in Sulfuric Acid Solution by Simultaneous Use of Oxygen and Cupric Ions
![The percentage of water of crystallisation in hydrated Copper Sulphate [CuSO4.5H2O] is:(Cu = 63.5, S = 32, O = 16, H = 1) The percentage of water of crystallisation in hydrated Copper Sulphate [CuSO4.5H2O] is:(Cu = 63.5, S = 32, O = 16, H = 1)](https://dwes9vv9u0550.cloudfront.net/images/5171946/16be827c-5f80-410b-a9f7-0243530e09a9.jpg)
The percentage of water of crystallisation in hydrated Copper Sulphate [CuSO4.5H2O] is:(Cu = 63.5, S = 32, O = 16, H = 1)
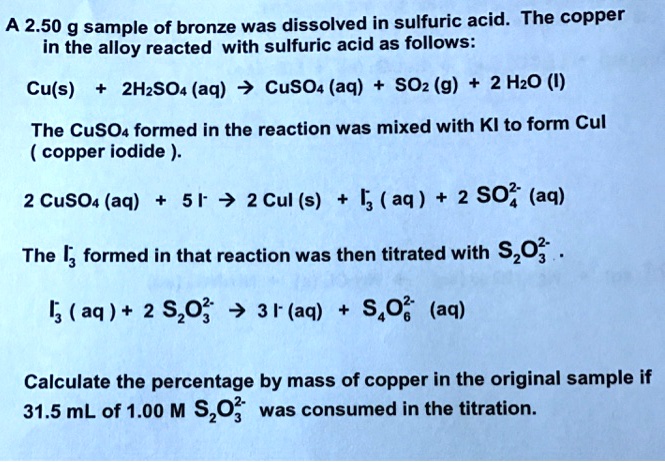

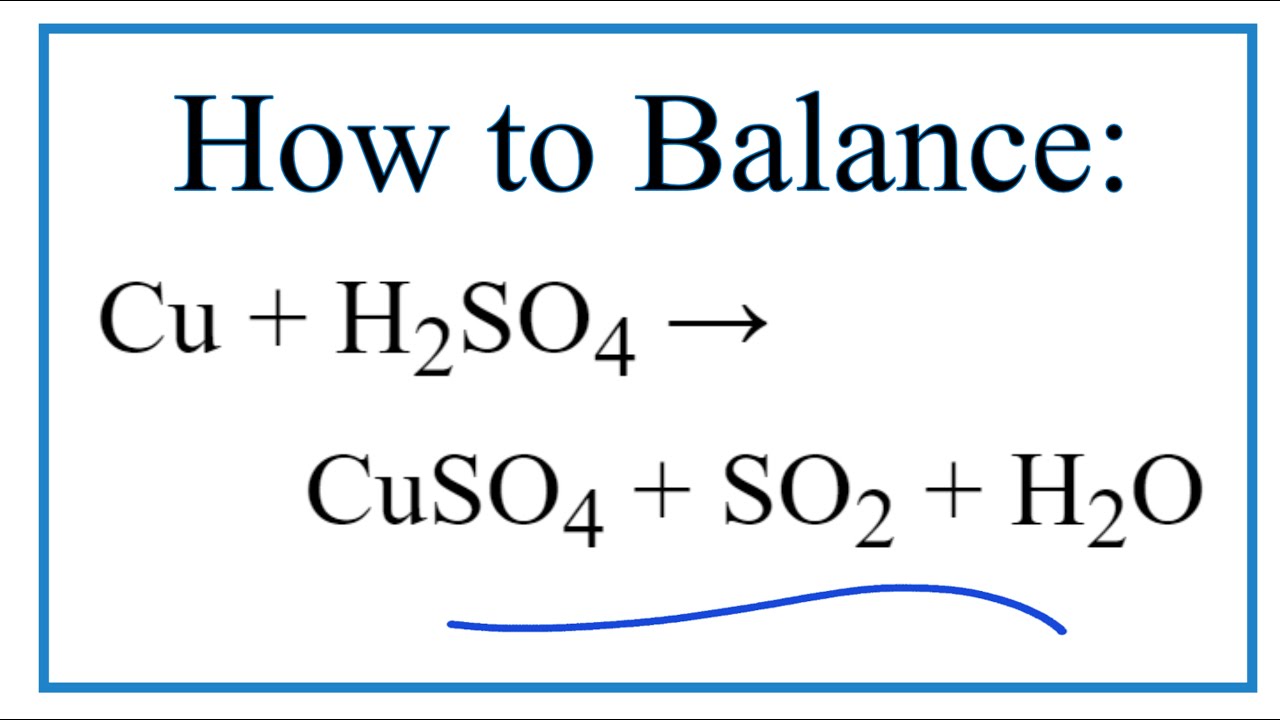


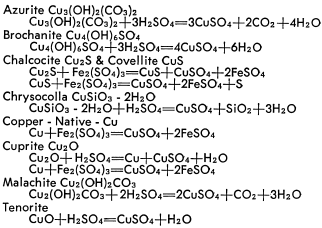


![PDF] Copper Dissoliution in Concentrated Sulfuric Acid | Semantic Scholar PDF] Copper Dissoliution in Concentrated Sulfuric Acid | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8c23bfe6f390e4c070ab06eca2828378bf1baac3/5-Table1-1.png)