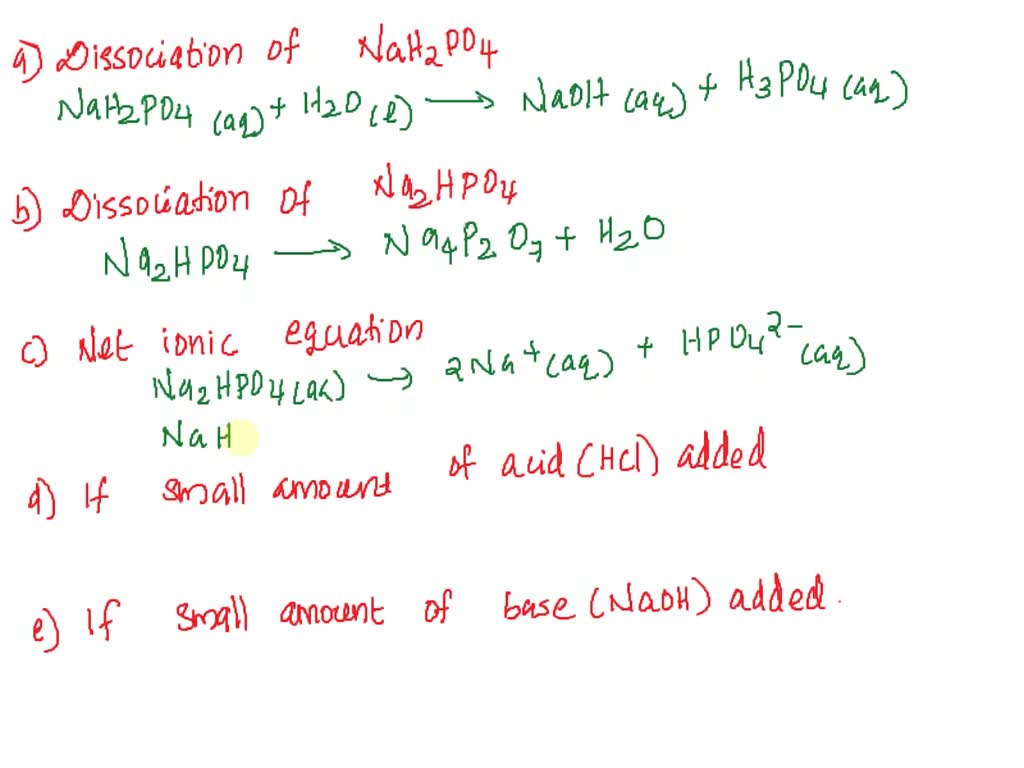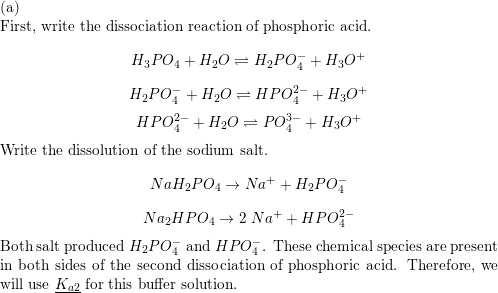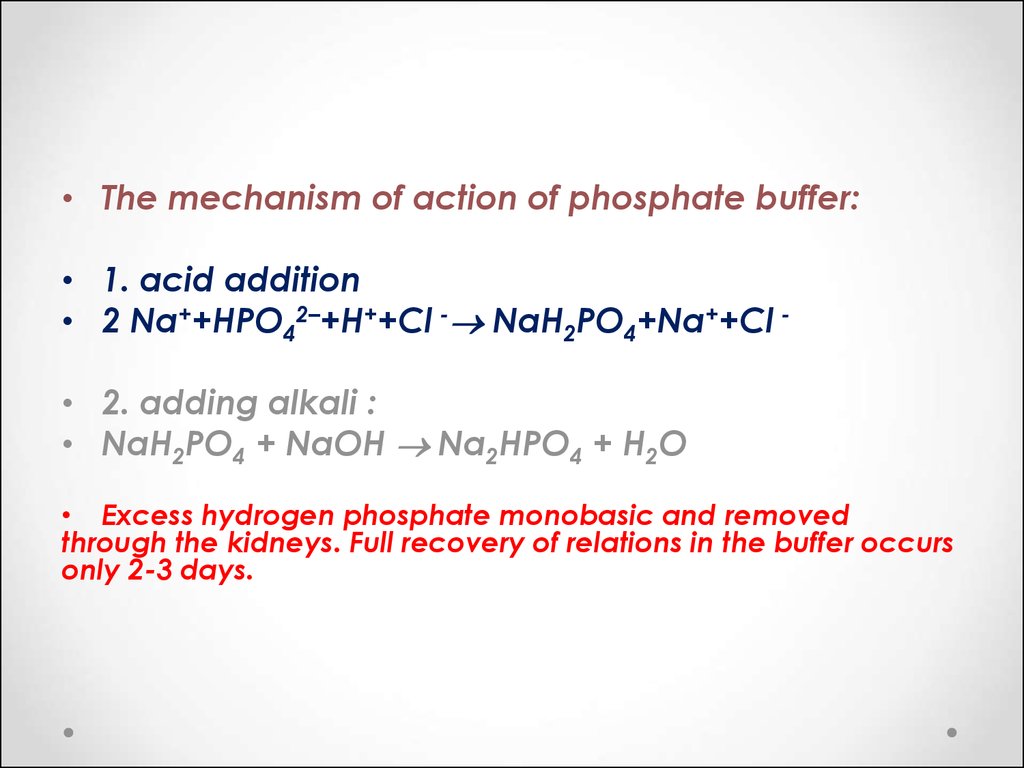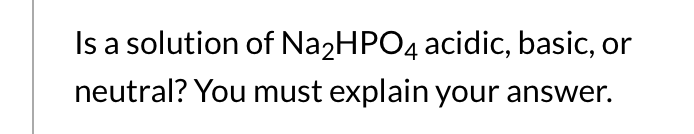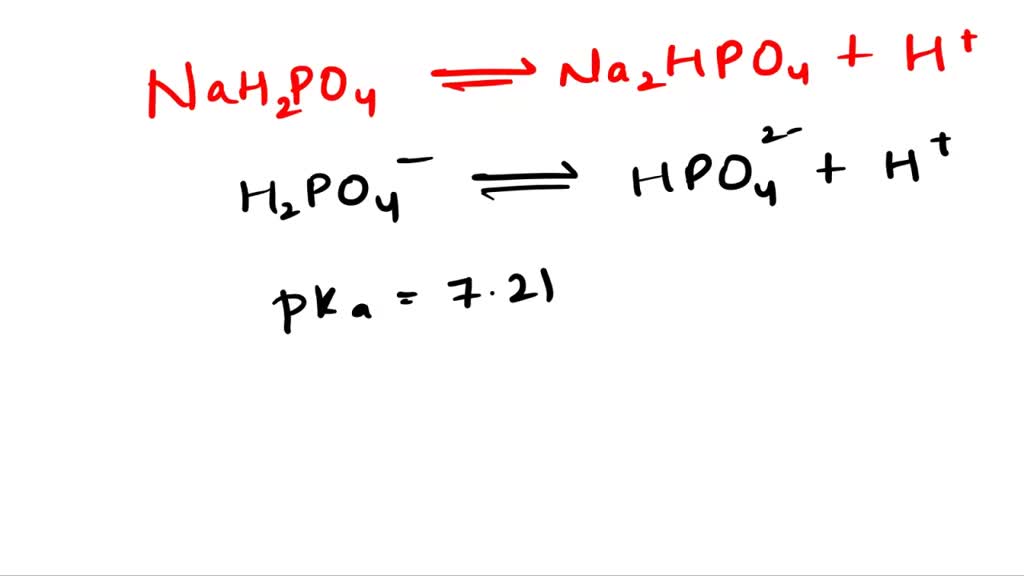
SOLVED: 1. Why is the equilibrium between the acid NaH2PO4, and its conjugate base Na2HPO4, a suitable buffer for maintaining intracellular pH (pH 6.9-7.3)?

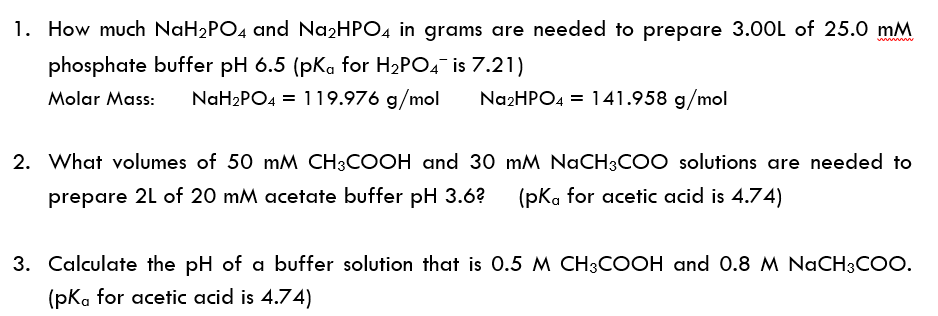
Finally, what mass of Na2HPO4 is required? Again, assume a 1.00 L volume buffer solution. Target pH = 7.49 - Brainly.com
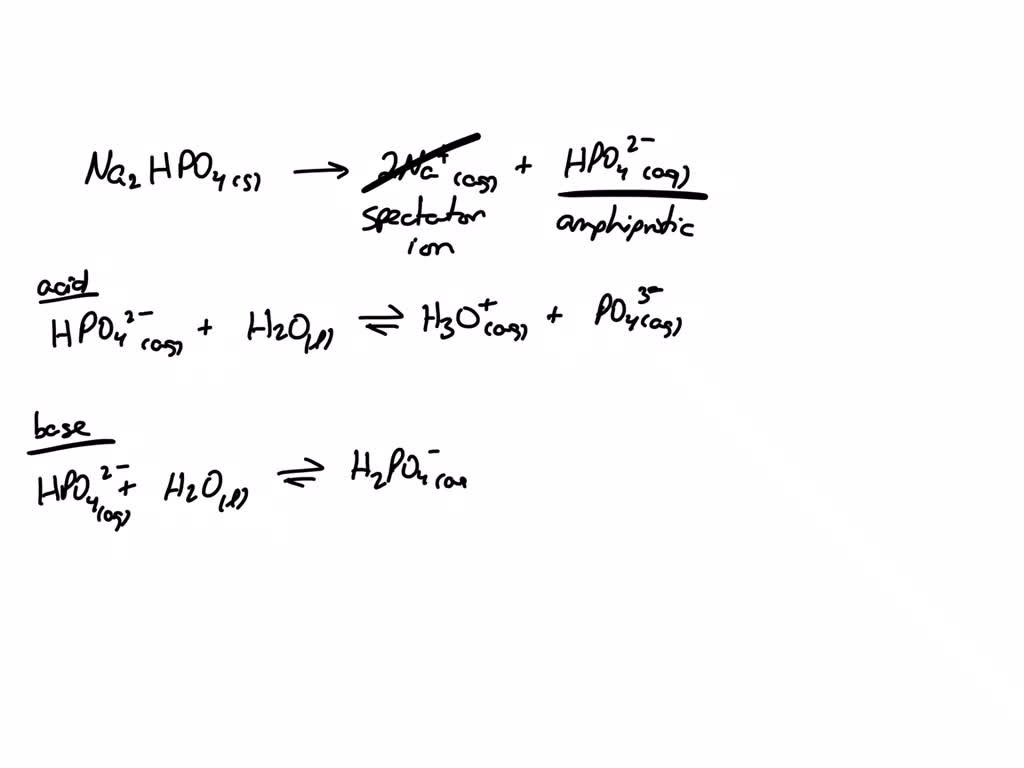
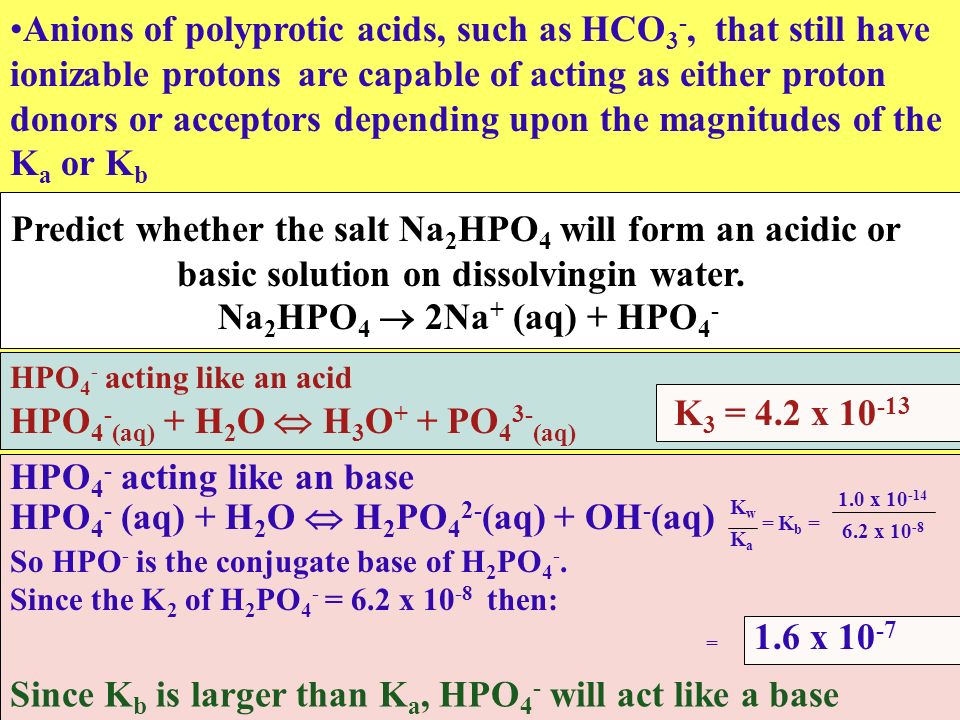
SOLVED: Predict whether the salt Na2HPO4 forms an acidic solution or a basic solution when dissolved in water.
Q. The equivalent mass of H3PO4 (Molecular weight = 98 g/mol) and Na2HPO4 (Molecular weight = 142 g/mol) in the reaction are respectively : H3PO4 + 2NaOH → Na2HPO4 + 2H2O (1) 49, 142 (2) 49, 71 (3) 98, 71 (4) 98, 142

A buffer solution 0.04 M in Na2HPO4 and 0.02 M in Na3PO4 is prepared. The electrolytic oxidation of 1.0 milli - mole of the organic compound RNHOH is carried out in 100
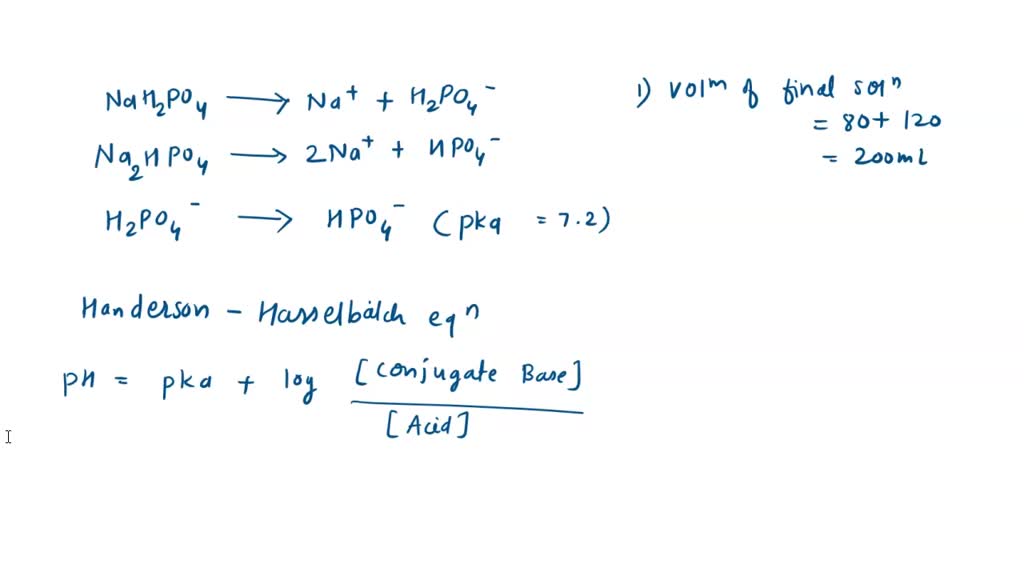
SOLVED: Calculate the pH of a buffer solution that is made by mixing 80 mL of 0.6 M NaH2PO4 with 120 mL of 0.8 M of Na2HPO4. Calculate the pH when 10

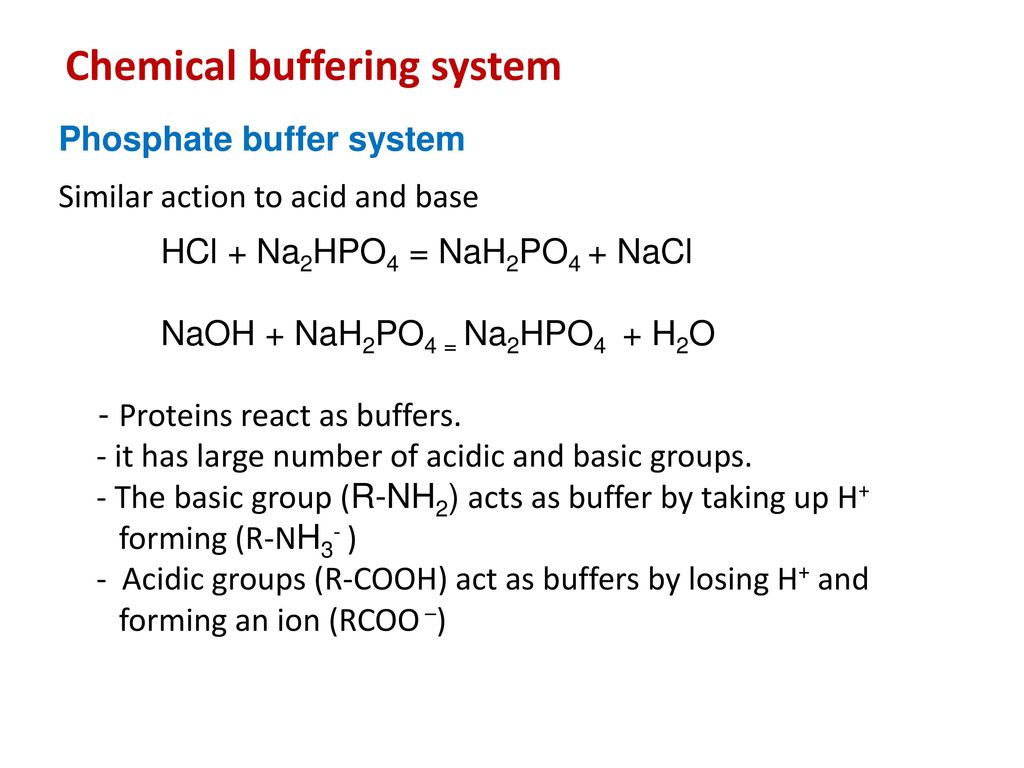
OneClass: 2. In the following reactions, label the acid, base, conjugate acid and conjugate base. (4 ...